Molecular profiling has improved prognostication and helped identify novel therapeutic targets in AML. Nonetheless, it has also created a complex web of molecular profiles and cytogenetic abnormalities and in many cases it remains unclear which specific alterations primarily drive leukemogenesis. We sought to review our patients (pts) treated with a hypomethylating agent (HMA) + Venetoclax (Ven), to evaluate molecular and cytogenetic profiles with their associated outcomes.
We identified 142 pts with AML treated with HMA + Ven at Northwell Health between January 2019 and December 2023. Through retrospective chart review, we obtained patient and disease characteristics, treatment regimens, clinical outcomes as well as molecular and cytogenetic profiling (using commercial AML panels) prior to the initiation of HMA + Ven. Bone marrow biopsy pathology reports were reviewed to identify the subtype of AML and grouped into De novo AML, AML with MDS related changes (AML-MRC) and therapy-related AML (t-AML). Median relapse free survival (mRFS) and median overall survival (mOS) in months (m) as defined by ELN 2022 (Dohner et al, Blood 2022), were estimated with Kaplan-Meier curves and compared utilizing log-rank testing. T-test was used to evaluate significant differences between categorical data.
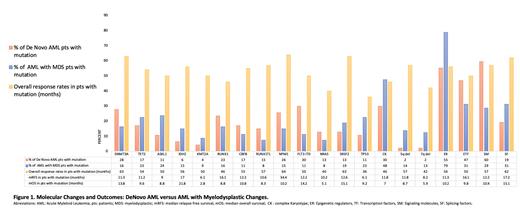
Of the 142 pts identified, the median age was 77. The majority of patients had adverse (n = 99) or intermediate risk (n = 31) genetics as defined by the 2022 ELN genetic risk classification. The overall response rate (ORR) was 42% with mOS of 9.6m. AML-MRC patients (n=80) had mOS of 9.1m and mRFS of 11.6m with 61 pts receiving HMA + Ven as frontline treatment. 47 pts with De novo AML had mOS of 13.4m and mRFS of 16.1m with 36 pts receiving HMA + Ven as frontline therapy. Only 15 pts had t-AML with mRFS of 12.3m and mOS of 8.8m with 10 pts receiving HMA + Ven as frontline therapy. There was no significant difference in mRFS or mOS between these groups, although there was a trend favoring De novo AML. 47 different molecular alterations were identified among the 142 patients. Figure 1 illustrates outcomes associated with the 16 most prevalent molecular alterations found in our AML population.
Mutational burden was similar between AML types (2.7 vs 3.0 vs 2.4 mutations per pt). De novo AML was associated with an increased mutation burden in signaling molecules (60%) and transcription factors (47%) compared to AML-MRC (29%, 31%). AML-MRC had a higher occurrence of mutations in epigenetic regulatory genes (79%) and splicing factors (31%) compared to De novo AML (55%, 19%). Mutations in splicing factors were associated with a significant increase in both mRFS and mOS(p = 0.01) compared to pts without mutations.
Pts with DNMT3A, NPM1 or SRSF2 mutations had the highest ORR to HMA + Ven (63%, 64% and 63% respectively). RUNX1, TP53, complex cytogenetics and del 7q were associated with worse ORR (46%, 36%, 46% and 42% respectively) as well as decreased mOS. SRSF2, mutated in both AML-MRC and De novo AML, not only had an elevated ORR, but improved mRFS and mOS. Contrarily, mRFS and mOS were not significantly improved despite the high ORR in DMT3A mutated pts. DNMT3A was mutated most often in AML-MRC and was associated with a higher rate of other epigenetic regulatory gene co-mutations. The same is true for patients with KMT2A mutations (ORR 50%, mOS 2.8m, mRFS 6.1m) which suggests ineffectiveness of HMA+ Ven in maintaining remissions with this alteration with possible early selection for resistant clones. RUNX1 mutations were associated with an improved mRFS (16.1m) despite lower mOS (8.8m). NPM1 mutations had the highest mRFS of 34.4m. The survival advantage may be secondary to the absence of adverse cytogenetics in 88% of these pts. IDH2 mutations was associated with a significantly improved mOS compared to unmutated IDH2; however this may be confounded by the use of an IDH2 inhibitor at relapse.
Our review of molecular alterations in AML pts identified several molecular alterations such as SRSF2, DNMT3A, RUNX1 which we found to be associated with favorable responses when treated with HMA + Ven. In addition, we demonstrated that NPM1 mutation remains favorable in patients treated with HMA + Ven. Our findings help contribute to the ever-growing, complex molecular and genomic profiling of AML patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal